Goal: Interrelate representations of kinematical quantities
Source: CT151.2-10
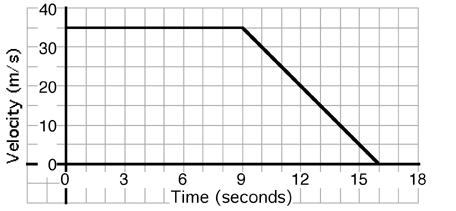
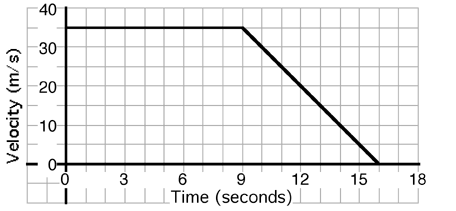
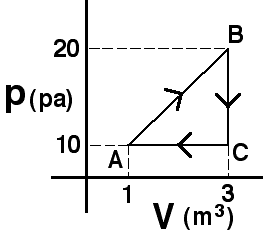
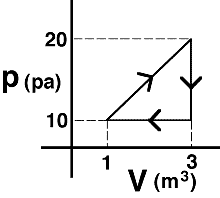
An object’s motion is described by the graph above. The displacement
of the object during the entire 16 seconds is most nearly…
- 200 meters
- 250 meters
- 300 meters
- 350 meters
- 400 meters
- 450 meters
- Cannot be determined
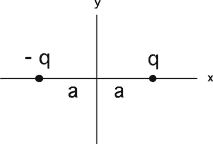
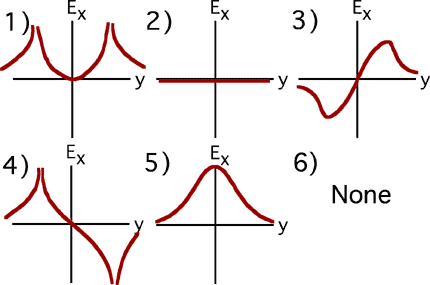




Commentary:
Answer
(7) Students have difficulty reading graphs and finding areas.