Goal: Reason with internal energy
Source: UMPERG-ctqpe194var

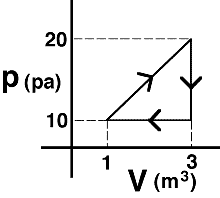
An
amount of an ideal gas is taken around the process shown. Which of the
following statements about the internal energy of the states is true?
- The internal energy of state B is twice that of state C.
- The internal energy of state B is equal to that of A and C combined.
- The internal energy of state A is half that of state C.
- The internal energy of state B is less than the internal energy of state
A. - none of the above
- cannot be determined


Commentary:
Answer
(1) Students need to know only that the internal energy depends
upon the product of p and V. Alternatively, they can reason that,
according to the Ideal Gas Law, this product is proportional to the
temperature and the temperature determines the internal energy