Goal: Hone the concept of work for a thermodynamic system
Source: UMPERG-ctqpe190
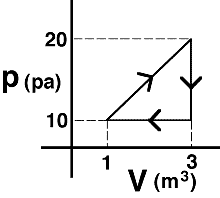
An
ideal gas is taken around the process shown. The net work done
on the gas is most nearly…
- 20 J
- -30 J
- 15 J
- -10 J
- none of the above
- cannot be determined
Goal: Hone the concept of work for a thermodynamic system
Source: UMPERG-ctqpe190

An
ideal gas is taken around the process shown. The net work done
on the gas is most nearly…
Commentary:
Answer
(4) The work done ON the system is the negative of the area of
the triangle. Students selecting answer #1 or #3 need to be sensitized
to the difference between work done on the gas versus by the gas.