Goal: Reasoning with thermodynamics
Source: ctqpe214

A
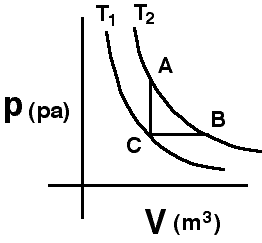
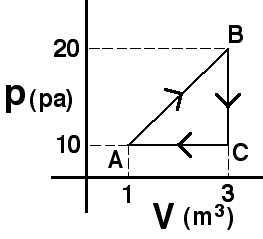
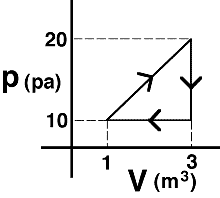
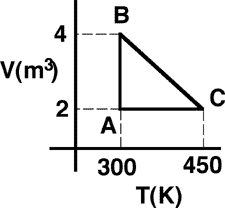
system consisting of a quantity of ideal gas has the two isotherms
shown. The system, initially at state C, can be taken along path CA to
final state A or along path CB to state B.
Which of the following is true:
- QCA < QCB
- QCA = QCB
- QCA > QCB
- Not enough information


Commentary:
Answer
(1) Since the internal energy of an ideal gas depends only on
temperature, states A and B have the same internal energy. Along path CB
the system does work requiring more heat to be added than along path CA.