Goal: Problem solving in thermodynamics
Source: UMPERG-ctqpe198
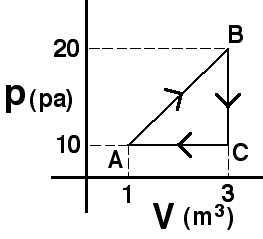
An
amount of an ideal gas is taken around the process shown.The amount of
heat extracted during process BC is
- 10 J
- 20 J
- 15 J
- 45 J
- 60 J
- none of the above
- cannot be determined
Goal: Problem solving in thermodynamics
Source: UMPERG-ctqpe198

An
amount of an ideal gas is taken around the process shown.The amount of
heat extracted during process BC is
Goal: Hone the concept of heat capacity
Source: UMPERG-ctqpe210

Two thermodynamic systems are made from the same material. The specific
heat of this material is independent of temperature. The bodies have
different masses and, initially, different temperatures as shown. If the
bodies are placed in thermal contact the final equilibrium temperature
is most nearly
(4) It is valuable to ask students how they obtained their answer. Each
of the offered answers is obtained by a common conceptual or algebraic
mistake.
Goal: Problem solving with thermal energy.
Source: UMPERG-ctqpe208
A cup of water is about 225 grams. The minimum amount of heat needed to
boil a cup of water at room temperature for tea is most nearly
(2) This problem requires that students know the specific heat of water
and assume that the water is initially at room temperature.
Goal: Hone the concept of heat and distinguish from internal energy.
Source: UMPERG-ctqpe191
Which of the following phrases best describes heat?
(4) Many students are confused about what heat is because the term is
not used consistently. ‘Heat’ is usually used to refer to the thermal
energy that flows into or out of a body. So ‘heat’ is not equivalent to
‘energy’ and it is inappropriate to refer to the heat possessed by a
body. Unfortunately, the redundant phrase ‘heat flow’ is often used in
texts. In addition, students frequently attribute any loss of coherent
energy to friction, which has converted the energy into ‘heat’.
Commentary:
Answer
(4) Since no work is done the change in internal energy must be
due to heat extraction. Some students may think that the answer cannot
be determined because they do not know the number of moles. These are
likely thinking that they need to find the temperature at each state to
answer the question.